Articles from Fresenius Kabi

Fresenius Kabi, an operating company of Fresenius, announced today that Epinephrine Injection, USP, is now available in the United States in 30 mg per 30 mL multi-dose vials.
By Fresenius Kabi · Via Business Wire · May 20, 2025

Fresenius Kabi, an operating company of Fresenius, and Formycon AG, announced today that the U.S. Food and Drug Administration (FDA) designated Otulfi® (ustekinumab-aauz) as an interchangeable biosimilar to the reference product Stelara® (ustekinumab).
By Fresenius Kabi · Via Business Wire · May 19, 2025

Fresenius Kabi, an operating company of Fresenius, announced today that the Centers for Medicare and Medicaid Services (CMS) issued a permanent, product-specific billing code for Otulfi® (ustekinumab-aauz).
By Fresenius Kabi · Via Business Wire · April 28, 2025

Fresenius announced that the Biologics License Application (BLA) for the denosumab biosimilars Conexxence® (denosumab-bnht) and Bomyntra® (denosumab-bnht) of its operating company Fresenius Kabi, has been approved by the U.S. Food and Drug Administration (FDA). These denosumab biosimilars are approved for all indications of the reference products: Prolia® (denosumab) and Xgeva® (denosumab), respectively. In addition, the Fresenius operating company has reached a global settlement with Amgen concerning its denosumab biosimilars.
By Fresenius Kabi · Via Business Wire · March 27, 2025

Fresenius Kabi, an operating company of Fresenius, and Formycon AG, a leading, independent developer of high-quality biosimilars, announced today that the ustekinumab biosimilar Otulfi® (ustekinumab-aauz) developed by Formycon AG, is now available in the United States. Otulfi® is an ustekinumab biosimilar for the reference product Stelara® (ustekinumab).
By Fresenius Kabi · Via Business Wire · March 3, 2025

Fresenius Kabi, an operating company of Fresenius, announced today it has introduced Calcitonin Salmon Injection, USP Synthetic, a calcium regulator, for the treatment of symptomatic Paget’s disease of the bone and hypercalcemia. The drug is also used to treat postmenopausal osteoporosis when alternative treatments are not suitable. Fresenius Kabi Calcitonin Salmon Injection is formulated, filled and packaged in the United States.
By Fresenius Kabi · Via Business Wire · February 10, 2025

Fresenius Kabi, an operating company of Fresenius, specializing in lifesaving medicines and technologies, announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its Adaptive Nomogram - an alternate algorithm that will be available in the Aurora Xi Plasmapheresis System - designed to optimize plasma collection efficiency.
By Fresenius Kabi · Via Business Wire · January 28, 2025
Fresenius Kabi, an operating company of Fresenius, announced today that Epinephrine Injection, USP, is now available in the United States as the first generic version of Epinephrine in a 1mg/1mL vial for U.S. customers.
By Fresenius Kabi · Via Business Wire · December 17, 2024

Fresenius Kabi, an operating company of Fresenius, announced today it has submitted a 510(k) notification to the U.S. Food and Drug Administration seeking clearance of the Aurora Xi Plasmapheresis System software version 2.0.
By Fresenius Kabi · Via Business Wire · November 21, 2024

Fresenius Kabi, an operating company of Fresenius, announced today it received a Novaplus Program Excellence Award from Vizient, Inc., the nation’s largest provider-driven healthcare performance improvement company. The recognition was announced at the Vizient Connections Summit in Las Vegas.
By Fresenius Kabi · Via Business Wire · November 14, 2024

Fresenius Kabi, an operating company of Fresenius, announced today that the Centers for Medicare and Medicaid Services (CMS) issued a permanent, product-specific billing code and granted pass-through payment status for Tyenne® (tocilizumab-aazg).
By Fresenius Kabi · Via Business Wire · October 29, 2024

Fresenius Kabi, an operating company of Fresenius, today named 12 new inductees into the National Blood Donation Hall of Fame. Nominated by blood centers across the U.S., the honorees were chosen for their extraordinary commitment to donating blood or for encouraging blood donation.
By Fresenius Kabi · Via Business Wire · October 17, 2024

Fresenius Kabi, an operating company of Fresenius, specializing in biopharmaceuticals, clinical nutrition, medical technologies, and I.V. generic drugs for critical and chronic conditions, and Formycon AG, a leading, independent developer of high-quality biosimilars, announced today that the United States (U.S.) Food and Drug Administration (FDA) has approved Otulfi™ (ustekinumab-aauz), its ustekinumab biosimilar referencing Stelara®** (ustekinumab). Otulfi™ is approved for the treatment of Crohn’s disease, ulcerative colitis, moderate to severe plaque psoriasis and active psoriatic arthritis. Fresenius Kabi is further continuing its momentum, striving at expanding its strong Biopharma platform, which is a substantial cornerstone of #FutureFresenius.
By Fresenius Kabi · Via Business Wire · September 30, 2024

Fresenius Kabi, an operating company of Fresenius, and the Association for the Advancement of Blood & Biotherapies (AABB) announced today that the 19th annual Blood Collectors Week is underway and runs from September 1 through September 7, 2024. Throughout the week, dedicated individuals who make blood collection possible – including phlebotomists, apheresis operators, medical directors, donor recruiters, technicians, and drivers – are honored for their vital role as the connection between blood donors and patients relying on blood for optimal medical treatment.
By Fresenius Kabi · Via Business Wire · September 1, 2024

Fresenius Kabi announced today it has launched Cetrorelix Acetate for Injection kit, an FDA-approved, cost-effective, generic option for women’s reproductive health. Cetrorelix Acetate for Injection is available immediately from Fresenius Kabi in a kit that contains one single-dose vial (0.25 mg cetrorelix), one pre-filled syringe with 1 mL sterile water for injection, one 20-gauge needle, and one 27-gauge needle.
By Fresenius Kabi · Via Business Wire · August 27, 2024

Fresenius Kabi, an operating company of Fresenius, has received the 2024 Trailblazer Award from Premier Inc., a leading technology-driven health care improvement company. Premier members comprise approximately 4,350 U.S. hospitals, health systems and more than 300,000 other providers and organizations in health care.
By Fresenius Kabi · Via Business Wire · August 5, 2024

Fresenius Kabi announced today that Arunesh Verma has joined the company as president of Fresenius Kabi USA and a member of the company’s global Executive Leadership Team, reporting to Pierluigi Antonelli, CEO of Fresenius Kabi.
By Fresenius Kabi · Via Business Wire · July 10, 2024
Fresenius, via its operating company Fresenius Kabi, announced today the immediate availability in the U.S. of its biosimilar Tyenne® (tocilizumab-aazg), in a subcutaneous formulation, which will increase access to affordable and cost-effective treatment options for use in the treatment of chronic autoimmune diseases.
By Fresenius Kabi · Via Business Wire · July 2, 2024

Angels for Change, a global non-profit on a mission to end drug shortages, presented the organization’s 2024 Drug Shortage Guardian Award to Fresenius Kabi USA, a health care company that specializes in lifesaving medicines and technologies for infusion, transfusion, and clinical nutrition.
By Fresenius Kabi · Via Business Wire · June 6, 2024

Fresenius, via its operating company Fresenius Kabi, announced today the immediate U.S. availability of Tyenne® (tocilizumab-aazg), a biosimilar of Actemra® (tocilizumab). Tyenne®, for use in the treatment of chronic autoimmune diseases, is available in an intravenous (IV) formulation.
By Fresenius Kabi · Via Business Wire · April 15, 2024

Fresenius Kabi announced today it has introduced Cyclophosphamide for Injection, USP, a generic substitute for Cytoxan, for use in treating several forms of cancer. Now available in the U.S., Cyclophosphamide for Injection, USP is the newest addition to Fresenius Kabi’s broad portfolio of generic oncology injectables that help make cancer therapies more affordable and accessible.
By Fresenius Kabi · Via Business Wire · February 28, 2024
Fresenius Kabi announced today it has introduced Posaconazole Injection, a generic substitute for Noxafil®, for use in treating or preventing serious fungal infections in adults and children who have an increased chance of getting these infections due to a weakened immune system. Now available in the U.S., Posaconazole Injection is the newest addition to Fresenius Kabi’s portfolio of more than 30 anti-infective molecules.
By Fresenius Kabi · Via Business Wire · January 29, 2024
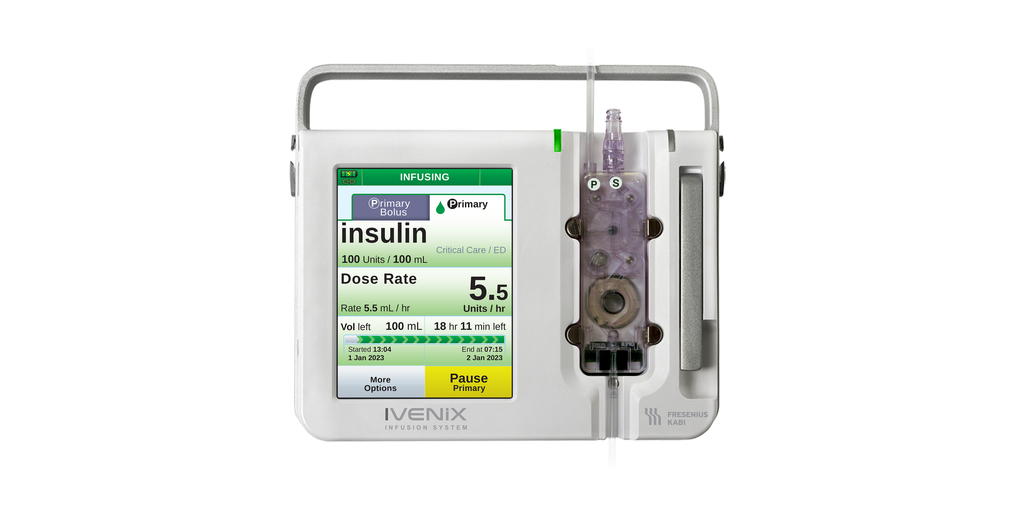
Fresenius Kabi announced today it has signed a multiyear agreement under which the Mayo Clinic is expected to purchase 10,000 Ivenix® large-volume infusion pumps for its hospitals and clinics in Minnesota, Arizona and Florida.
By Fresenius Kabi · Via Business Wire · December 14, 2023

Fresenius Kabi marked the 25th anniversary of its partnership with blood donation centers by naming the 12 newest inductees into the Fresenius Kabi National Blood Donation Hall of Fame. The latest honorees were nominated by blood centers across the United States for their exemplary commitment to donating blood and/or encouraging blood donation.
By Fresenius Kabi · Via Business Wire · October 10, 2023
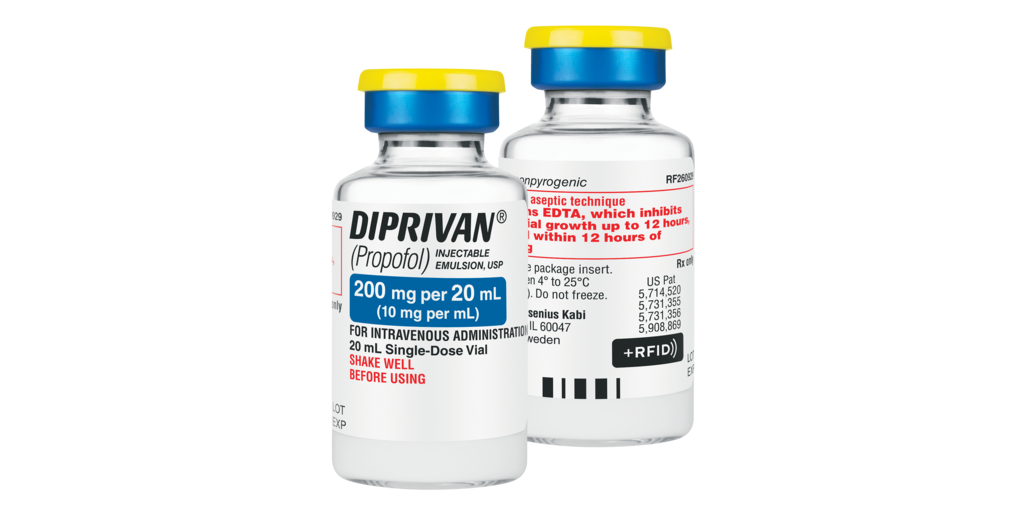
Fresenius Kabi announced today it has introduced +RFID smart labels for Diprivan® (Propofol) Injectable Emulsion, USP, 200 mg per 20 mL in single-dose vials, sold in the United States. The +RFID labels are now fully compatible with all major RFID kit and tray systems in the U.S.
By Fresenius Kabi · Via Business Wire · October 9, 2023

Fresenius Kabi has been named the 2023 Supplier Partner of the Year by Vizient, Inc., the nation’s largest provider-driven health care performance improvement company. The recognition was celebrated September 18-21 at the 2023 Vizient Connections Summit in Las Vegas.
By Fresenius Kabi · Via Business Wire · October 3, 2023

Fresenius Kabi has been selected to exhibit its Ivenix Infusion System at the Vizient Innovative Technology Exchange. Vizient, Inc, the nation’s largest provider-driven healthcare performance improvement company, will hold the Exchange on Oct. 3 in Grapevine, Texas.
By Fresenius Kabi · Via Business Wire · October 2, 2023

Fresenius Kabi announced today it has signed an agreement with Virginia Oncology Associates (VOA) under which VOA will purchase the Ivenix Infusion System to deliver medications for its patients. VOA specializes in treating people with cancer and blood disorders by providing access to innovative therapies and technologies.
By Fresenius Kabi · Via Business Wire · September 14, 2023

Fresenius Kabi and Lupagen Inc. announced today the companies have entered into a development and supply agreement for technologies designed to bring the delivery of cell and gene therapies to the bedside.
By Fresenius Kabi · Via Business Wire · August 24, 2023

Fresenius Kabi announced today it has introduced Plerixafor Injection, a generic equivalent to Mozobil®, available immediately in the United States in a 24 mg per 1.2 mL single dose vial.
By Fresenius Kabi · Via Business Wire · August 9, 2023

Fresenius Kabi announced today it has launched Gadobutrol Injection, a generic substitute for the contrast agent Gadavist®, which is used in magnetic resonance imaging (MRI) procedures.
By Fresenius Kabi · Via Business Wire · August 8, 2023
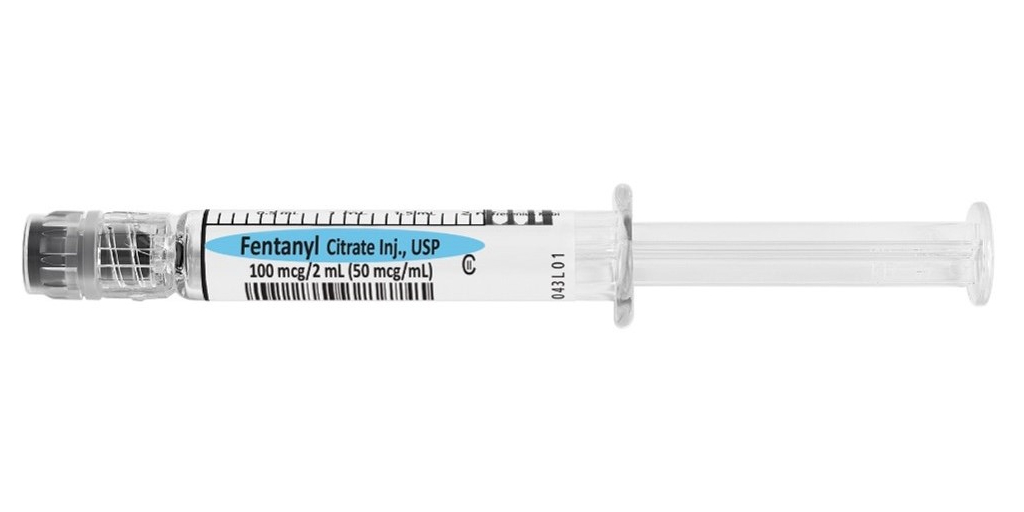
Fresenius Kabi announced today the launch and immediate availability in the U.S. of Fentanyl Citrate Injection, USP in 100 mcg per 2 mL Simplist® ready-to-administer prefilled syringes, the only 100 mcg per 2 mL presentation available on the market in a manufacturer-prepared prefilled syringe.
By Fresenius Kabi · Via Business Wire · July 11, 2023
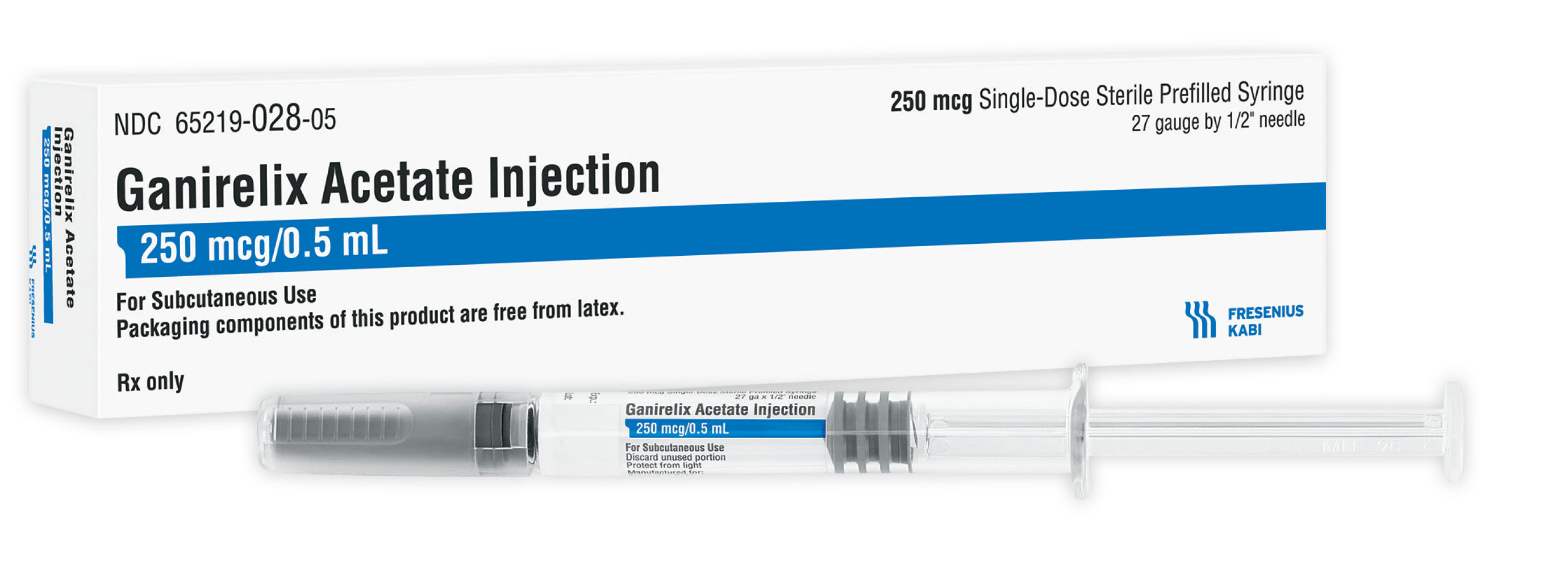
Fresenius Kabi announced today it has launched Ganirelix Acetate Injection, a generic fertility drug, as part of the company’s expansion in women’s health. Ganirelix Acetate Injection is available immediately from Fresenius Kabi in 250 mcg/0.5 mL prefilled syringes.
By Fresenius Kabi · Via Business Wire · July 10, 2023

Fresenius Kabi announced today the immediate availability in the U.S. of its citrate-free adalimumab biosimilar IDACIO® (adalimumab-aacf) for use in the treatment of chronic autoimmune diseases for all eligible indications of the reference product, Humira® (adalimumab). IDACIO® is available in a self-administered prefilled syringe and a self-administered pre-filled pen (autoinjector).
By Fresenius Kabi · Via Business Wire · July 3, 2023

Fresenius Kabi announced today that its Ivenix® Infusion System has been selected by the Metrodora Institute to meet the Institute’s infusion medicine needs. The Metrodora Institute is an integrated, multidisciplinary medical and research center dedicated to treating neuroimmune axis disorders.
By Fresenius Kabi · Via Business Wire · June 29, 2023

Fresenius Kabi, a leading provider of injectable medicines, announced today it has launched the diagnostic pharmaceutical agent, Sincalide for Injection, an authorized generic for Bracco Diagnostics Inc.’s Kinevac® (Sincalide for Injection) product. The new product broadens Fresenius Kabi’s radiology portfolio and is available immediately in the United States.
By Fresenius Kabi · Via Business Wire · June 21, 2023
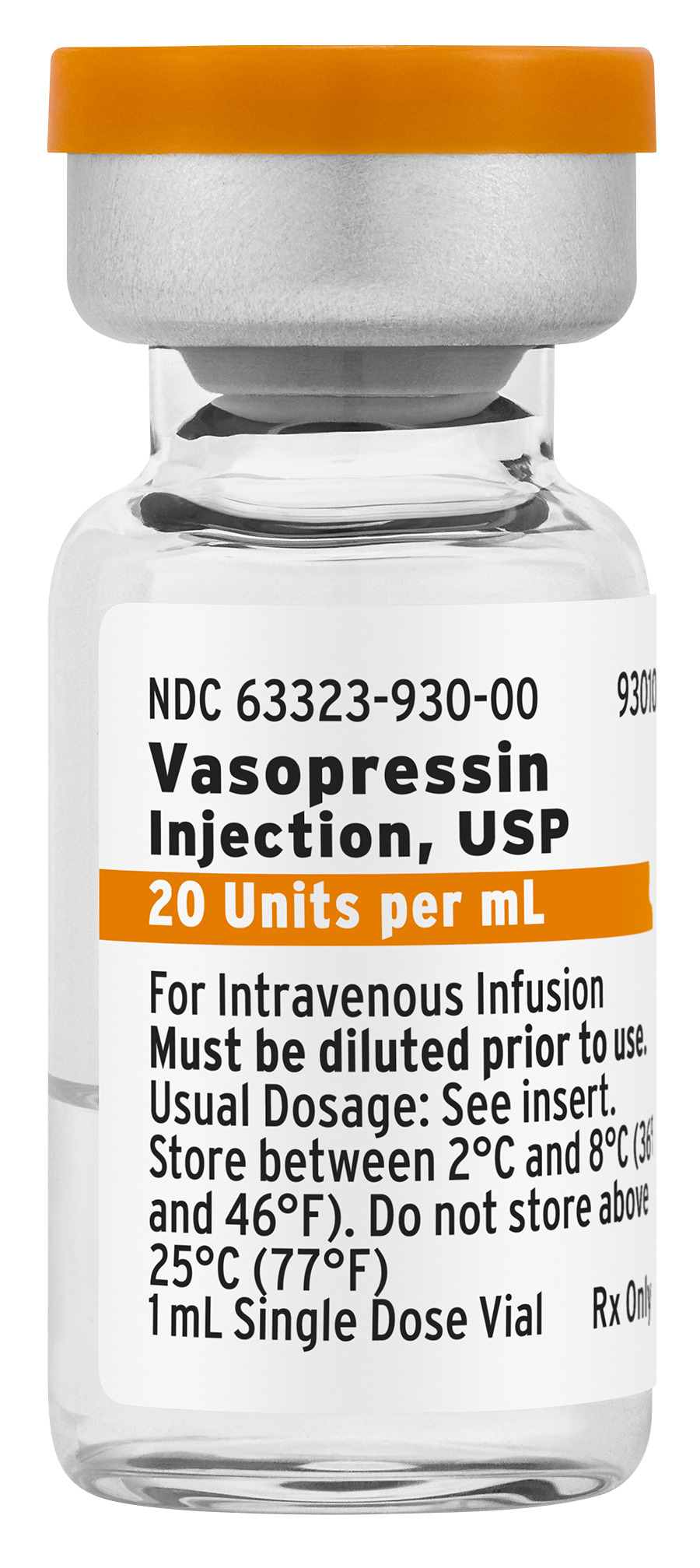
Fresenius Kabi announced today the availability in the United States of Vasopressin Injection, USP, a generic equivalent to Vasostrict®. Fresenius Kabi Vasopressin Injection, USP is an approved treatment option for adults with vasodilatory shock and is available in a 20 Units per 1 mL Single Dose Vial.
By Fresenius Kabi · Via Business Wire · June 14, 2023

Fresenius Kabi announced today the launch and immediate availability in the U.S. of Diazepam Injection, USP in 10 mg per 2 mL Simplist® ready-to-administer prefilled syringes.
By Fresenius Kabi · Via Business Wire · May 31, 2023
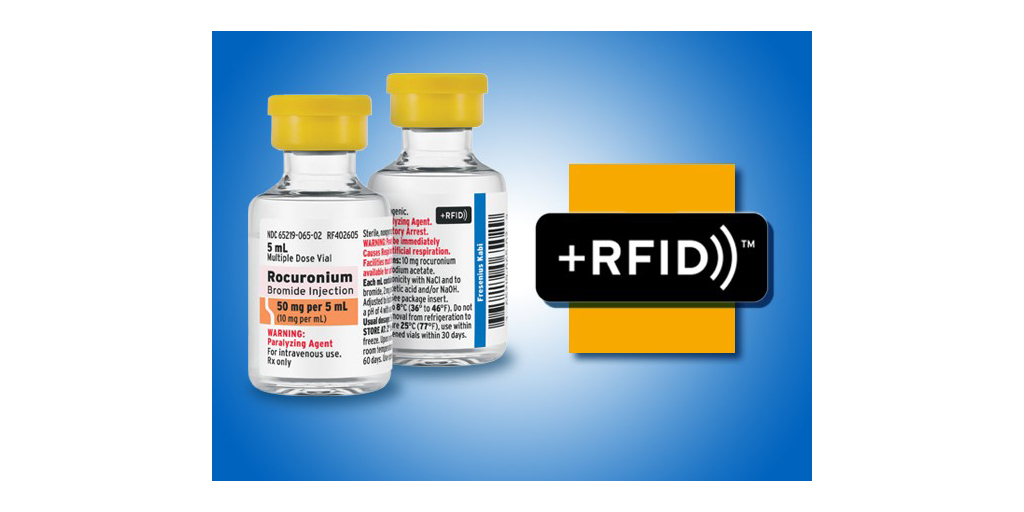
Fresenius Kabi, a leading provider of injectable medications, announced today it has launched Rocuronium Bromide Injection with advanced RFID-enabled labels in the United States. Rocuronium Bromide Injection is part of Fresenius Kabi's +RFID™ portfolio of smart-labeled medications, designed to help enhance patient safety and support a more-efficient medication inventory process. This will mark the first +RFID product that is compatible with Bluesight’s KitCheck product, the leading kit and tray solution for American hospitals allowing +RFID products to be used across all major RFID kit and tray vendors.
By Fresenius Kabi · Via Business Wire · May 30, 2023

Fresenius Kabi announced today it has been awarded a Breakthrough Technology group purchasing agreement with Premier, Inc. Effective May 1, 2023, the new agreement allows Premier members, at their discretion, to benefit from special pricing and terms pre-negotiated by Premier for the Ivenix Infusion System from Fresenius Kabi.
By Fresenius Kabi · Via Business Wire · May 23, 2023

Fresenius Kabi announced today that the Centers for Medicare and Medicaid Services (CMS) issued a permanent product-specific Q-code for Stimufend® (pegfilgrastim-fpgk). Under the Healthcare Common Procedure Coding System (HCPCS), the Q-code assigned to Stimufend is effective for patients administered Stimufend on and after April 1, 2023. The KABICARE™ patient services hub is available to facilitate successful access and reimbursement.
By Fresenius Kabi · Via Business Wire · May 16, 2023

Fresenius Kabi announced today its Ivenix Infusion System has received an Innovative Technology contract from Vizient, Inc. the nation's largest member-driven health care performance improvement company. Vizient members include more than half of all acute care hospitals in the United States, including 97 percent of U.S. academic medical centers.
By Fresenius Kabi · Via Business Wire · April 17, 2023
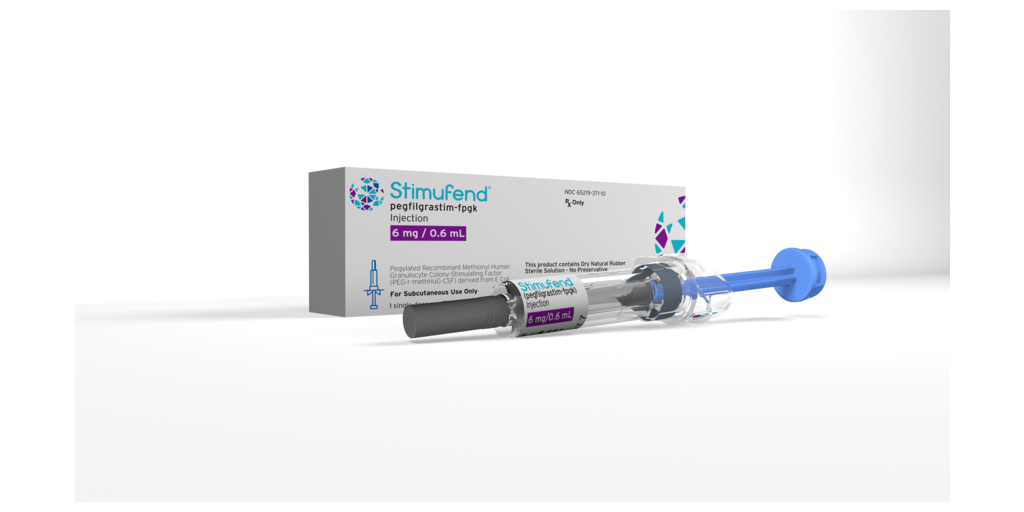
Fresenius Kabi announced today the immediate availability in the U.S. of Stimufend® (pegfilgrastim-fpgk), the company’s biosimilar to Neulasta® (pegfilgrastim), for use in patients at risk for febrile neutropenia, a common side effect of many anti-cancer medications. Stimufend is available in a single-dose, pre-filled syringe that delivers 6mg/0.6mL solution for subcutaneous injection.
By Fresenius Kabi · Via Business Wire · February 16, 2023

Fresenius Kabi announced today that its Ivenix Infusion System has been successfully implemented at San Luis Valley Health in Colorado.
By Fresenius Kabi · Via Business Wire · January 10, 2023

Fresenius Kabi announced today the immediate availability in the U.S. of Lacosamide Injection, USP, a generic equivalent to VIMPAT®. Fresenius Kabi Lacosamide Injection, USP is an approved treatment option for partial-onset seizures in patients 17 years of age and older and is available in 200 mg per 20 mL single-dose vials.
By Fresenius Kabi · Via Business Wire · December 15, 2022
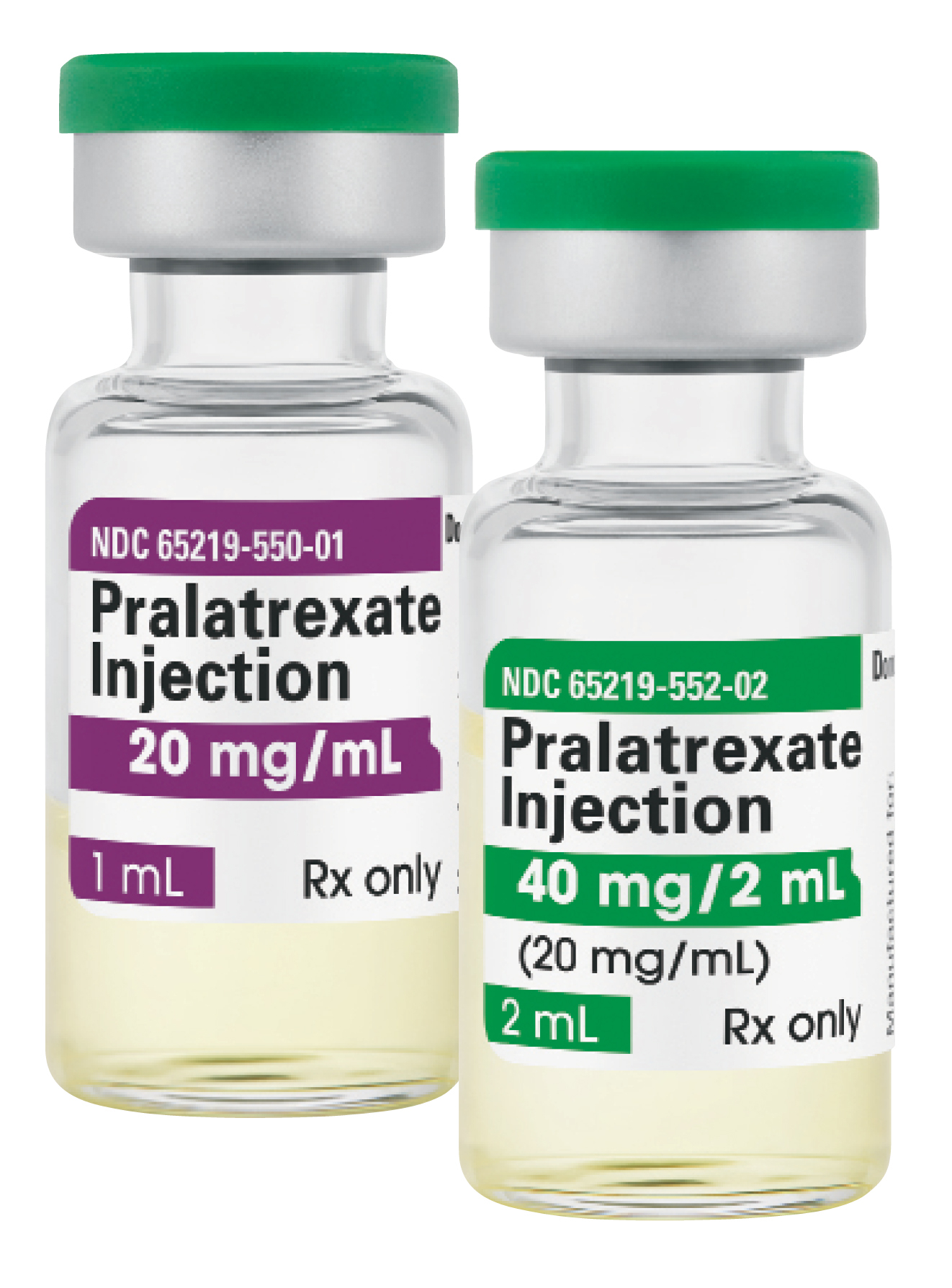
Fresenius Kabi announced today it has introduced Pralatrexate Injection, a generic equivalent to Folotyn®, for the treatment of relapsed or refractory peripheral T-cell lymphoma. Fresenius Kabi Pralatrexate Injection is available immediately in the United States and is the newest addition to the company’s injectable oncology medicine portfolio, the largest in U.S. health care.
By Fresenius Kabi · Via Business Wire · December 8, 2022

Fresenius Kabi announced today it has introduced KabiCare Nutrition Resources in the United States as part of its KabiCare patient support program. The KabiCare Nutrition Resources program provides support for insurance reimbursement questions including a helpline staffed by experts to provide billing and coding support, insurance support such as benefit investigation, prior authorization and claims appeals. More information about KabiCare Nutrition Resources can be found at kabicare.us.
By Fresenius Kabi · Via Business Wire · December 5, 2022

Fresenius Kabi today announced the 12 newest inductees into the Fresenius Kabi Blood Donation Hall of Fame. For 24 years, the Hall of Fame has recognized and shared the unique stories of those who are passionate about and committed to blood donation.
By Fresenius Kabi · Via Business Wire · November 9, 2022

Fresenius Kabi announced today the successful smart pump interoperability of its Ivenix Infusion System at Fort HealthCare, a regional health care provider in Wisconsin.
By Fresenius Kabi · Via Business Wire · October 11, 2022

Fresenius Kabi has been named the 2022 Pharmaceutical Supplier Partner of the Year by Vizient, Inc., the nation’s largest member-driven health care performance improvement company. The recognition was September 19-21 at the 2022 Vizient Connections Summit in Las Vegas.
By Fresenius Kabi · Via Business Wire · October 6, 2022
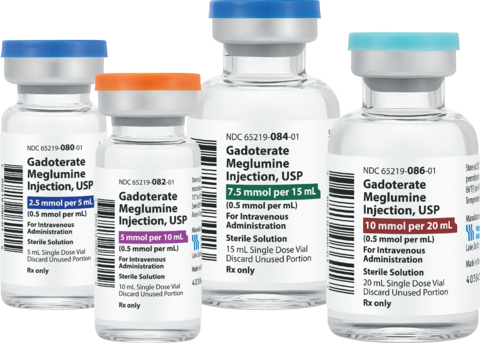
Fresenius Kabi announced today it has launched Gadoterate Meglumine Injection, USP, a bioequivalent and therapeutic equivalent substitute for the contrast agent Dotarem®. This is the second contrast agent introduced by Fresenius Kabi in the United States this year. Fresenius Kabi introduced Iodixanol Injection, USP in July during a nationwide shortage.
By Fresenius Kabi · Via Business Wire · October 4, 2022

Fresenius Kabi has been selected to exhibit the Ivenix Infusion System at the Vizient Innovative Technology Exchange. Vizient, Inc, the nation’s largest member-driven health care performance improvement company, will hold the Exchange on October 17, 2022, in Dallas.
By Fresenius Kabi · Via Business Wire · September 13, 2022
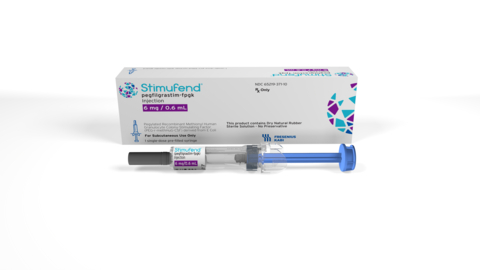
Fresenius Kabi, a global health care company that specializes in pharmaceuticals, medical technologies, and nutrition products for critical and chronic conditions, announced today that the United States (U.S.) Food and Drug Administration (FDA) has approved its biosimilar, Stimufend® (pegfilgrastim-fpgk), for use in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia. The company expects to launch the product in a prefilled syringe early next year and in an on-body injector following FDA approval.
By Fresenius Kabi · Via Business Wire · September 6, 2022

Fresenius Kabi and AABB announced today the official start of the 17th annual Blood Collectors Week celebration, which runs September 4-10, 2022.
By Fresenius Kabi · Via Business Wire · September 4, 2022
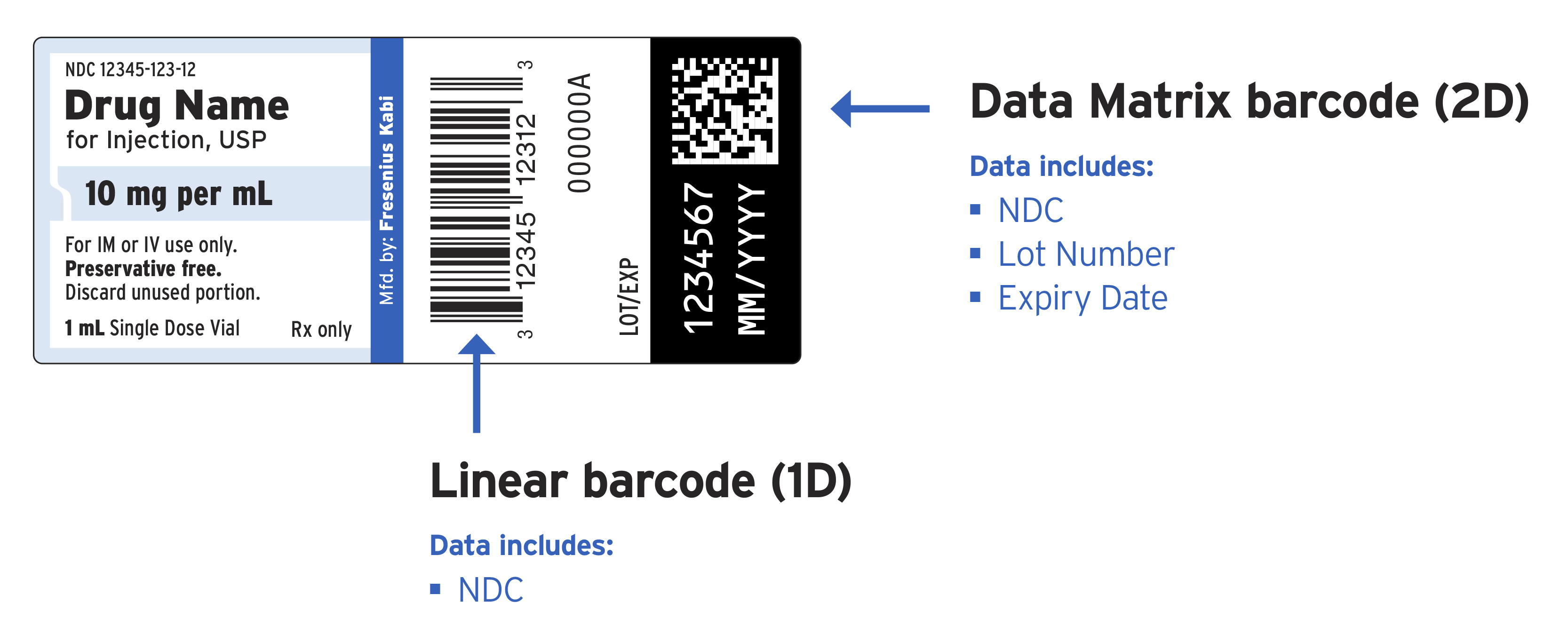
Fresenius Kabi announced today the introduction of a three-year plan to add GS1 Data Matrix barcodes to its pharmaceutical portfolio of vials, syringes, IV solutions and parenteral nutrition products at the unit of use. The initiative will help streamline workflows at health care facilities by reducing error-prone manual data entry by providing automatic identification and verification of products in medication management systems. Fresenius Kabi USA is the first pharmaceutical company to commit to offering GS1 Data Matrix barcodes on all drug product labels. The initiative was piloted on select IV products earlier this year and will continue with the first vial products available in early fall.
By Fresenius Kabi · Via Business Wire · August 8, 2022
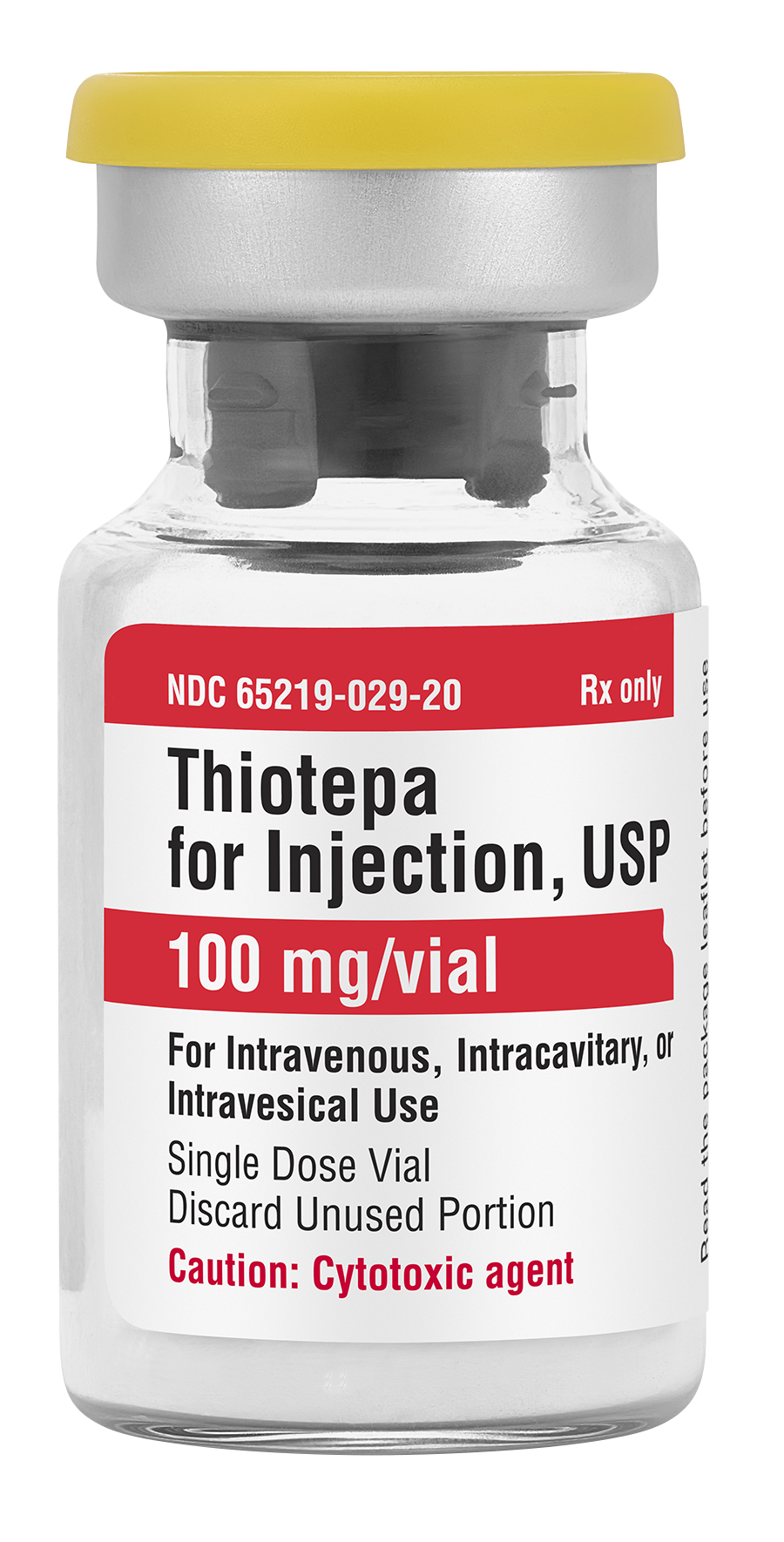
Fresenius Kabi announced today it has introduced Thiotepa for Injection, USP, in the United States. Thiotepa, a generic equivalent to Tepadina®, is the newest addition to the company’s injectable oncology portfolio – the most comprehensive in the industry.
By Fresenius Kabi · Via Business Wire · July 29, 2022

Fresenius Kabi announced today the immediate availability in the U.S. of Glycopyrrolate Injection, USP in 0.6 mg per 3 mL Simplist® ready-to-administer prefilled syringes.
By Fresenius Kabi · Via Business Wire · July 25, 2022
Fresenius Kabi announced today it will introduce a portfolio of generic contrast media agents in the United States, starting immediately with the launch of Iodixanol Injection, USP, a product the U.S. Food and Drug Administration (FDA) lists as being in shortage nationwide.
By Fresenius Kabi · Via Business Wire · July 18, 2022
Fresenius Kabi announced today the immediate availability in the United States of Romidepsin for Injection, the newest addition to the company’s broad portfolio of injectable oncology medicines.
By Fresenius Kabi · Via Business Wire · July 12, 2022

Fresenius Kabi announced today that its growing +RFID™ portfolio of radio frequency tagged medications is now compatible with AmerisourceBergen’s medication tray solution. AmerisourceBergen is a global health care company and one of the largest pharmaceutical distributors in the United States. AmerisourceBergen’s medication tray solution is designed to help hospitals improve medication inventory visibility and tracking using advanced Radio Frequency Identification (RFID) technology.
By Fresenius Kabi · Via Business Wire · June 14, 2022
Fresenius Kabi announced today the immediate availability in the U.S. of PEMEtrexed for Injection, USP, a new generic equivalent to Alimta®. Fresenius Kabi produces its PEMEtrexed for Injection, USP in the United States, where the company offers the most comprehensive injectable oncology portfolio of any pharmaceutical manufacturer.
By Fresenius Kabi · Via Business Wire · May 31, 2022

Fresenius Kabi announced today that Brandee Pappalardo, Ph.D., M.P.H. has joined the company as senior vice president and Chief Medical Officer for North America. Dr. Pappalardo reports to John Ducker, president and CEO of Fresenius Kabi in North America and is a member of the company’s North America executive committee.
By Fresenius Kabi · Via Business Wire · May 18, 2022
Fresenius Kabi announced today it has introduced Bortezomib for Injection, a new generic equivalent to Velcade® in the U.S. and the newest addition to the most comprehensive injectable oncology portfolio in the industry.
By Fresenius Kabi · Via Business Wire · May 2, 2022
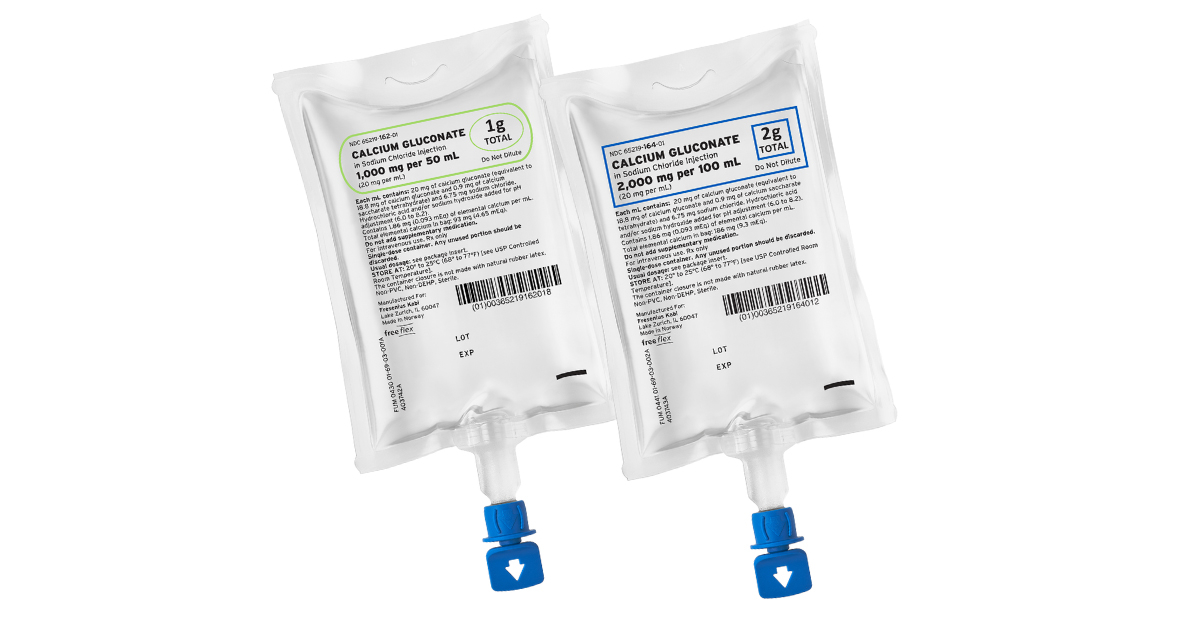
Fresenius Kabi announced today it has introduced two new presentations of Calcium Gluconate in Sodium Chloride Injection in the United States in the company’s proprietary, ready-to-administer freeflex® containers.
By Fresenius Kabi · Via Business Wire · April 27, 2022

Blood Centers of America (BCA), America’s Blood Centers (ABC) and Fresenius Kabi announced today that the three organizations have joined together on a nationwide campaign in the United States to provide needed blood-collection and transfusion supplies to those impacted in Ukraine.
By Fresenius Kabi · Via Business Wire · April 18, 2022

Fresenius Kabi announced today it has introduced KabiConnect, part of the KabiCare patient support program in the United States, to offer copay assistance for the company’s generic oncology medicines. Fresenius Kabi offers the most comprehensive portfolio of generic injectable oncology medicines in the U.S.
By Fresenius Kabi · Via Business Wire · April 13, 2022

Fresenius Kabi announced today it has been granted an expanded indication for SMOFlipid® Lipid Injectable Emulsion (ILE) for pediatric patients in the United States, including term and preterm neonates, making it the first and only four-oil lipid emulsion for parenteral nutrition patients of every age.1 SMOFlipid can be used throughout the continuum of care – from the hospital to home care settings.
By Fresenius Kabi · Via Business Wire · March 24, 2022
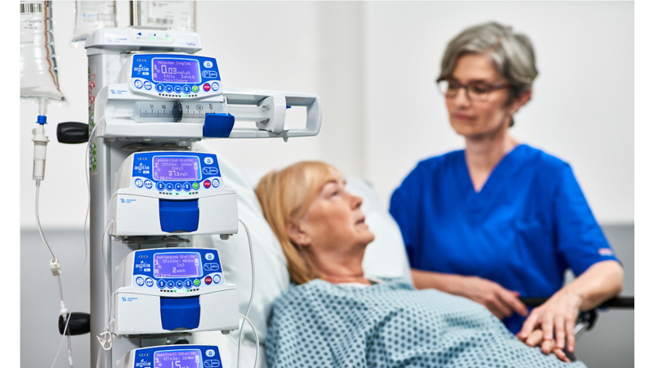
Fresenius Kabi, a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition, announced today it has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA) for its wireless Agilia® Connect Infusion System which includes the Agilia® Volumetric Pump and the Agilia® Syringe Pump with Vigilant® Software Suite-Vigilant® Master Med technology. The Agilia Connect volumetric pump and syringe pump are the first to be cleared by following TIR101 standards, which were developed by the Association for the Advancement of Medical Instrumentation (AAMI) in 2021.
By Fresenius Kabi · Via Business Wire · March 14, 2022

Fresenius Kabi announced today it inducted 12 people and groups as the 2021 class of the Fresenius Kabi Blood Donation Hall of Fame. The nationwide program recognizes and shares the unique stories of people who are passionate about and committed to blood donation.
By Fresenius Kabi · Via Business Wire · November 9, 2021

Fresenius Kabi and Health Care Logistics®, Inc. (HCL) today announced a collaboration designed to make drug replenishment in hospitals more efficient and improve patient safety throughout the medication-use process. The new collaboration allows customers to utilize Fresenius Kabi’s +RFID™ smart-labeled medications with HCL’s Stat Stock® inventory control solution.
By Fresenius Kabi · Via Business Wire · September 30, 2021

Fresenius Kabi announced today the U.S. introduction of Fentanyl Citrate Injection, USP 50 mcg per 1 mL in its proprietary Simplist® ready-to-administer prefilled syringe, the only 1 mL presentation available in the U.S. today. The 50 mcg per 1 mL prefilled syringe is designed to support initiatives at hospitals nationwide to reduce waste and diversion and help ensure safe delivery of the medication by eliminating steps where errors can occur.
By Fresenius Kabi · Via Business Wire · July 6, 2021

Fresenius Kabi, a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition, announced today it has received a 2021 Supplier Legacy Award from Premier Inc. (www.premierinc.com), a leading health care improvement company.
By Fresenius Kabi · Via Business Wire · June 21, 2021
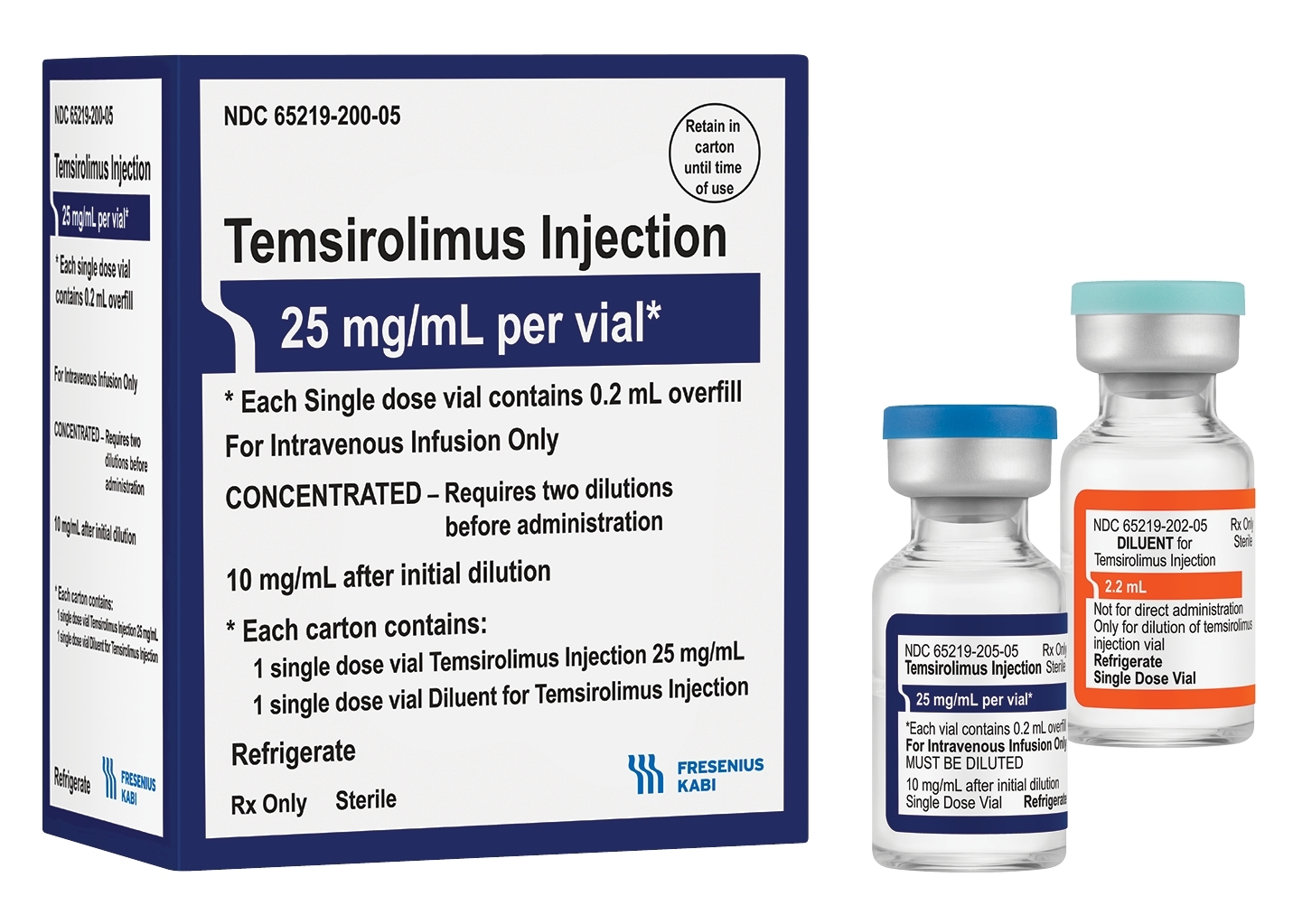
Fresenius Kabi announced today the immediate availability of Temsirolimus Injection in the United States. Fresenius Kabi’s Temsirolimus Injection is supplied as a kit including one vial of 25 mg/mL Temsirolimus solution and one vial of diluent.
By Fresenius Kabi · Via Business Wire · May 21, 2021
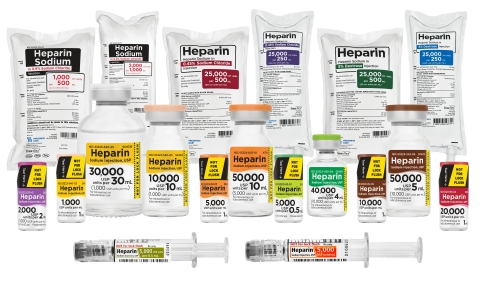
Fresenius Kabi announced today it has introduced in the United States two new presentations of Heparin Sodium in convenient, ready-to-administer Freeflex® IV bags.
By Fresenius Kabi · Via Business Wire · April 21, 2021